Anion-Selective Redox Electrodes: Electrochemically Mediated Separation with Heterogeneous Organometallic Interfaces
Abstract
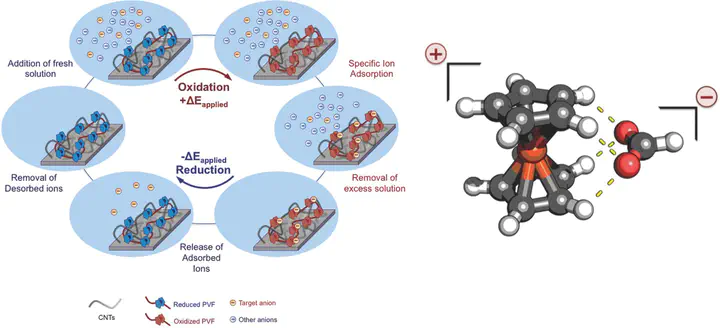
Redox species have been explored extensively for catalysis, energy storage, and molecular recognition. It is shown that nanostructured pseudocapacitive electrodes functionalized with ferrocene-based redox polymers are an attractive platform for the selective sorptive separation of dilute organic anions from strong aqueous and organic electrolyte solutions, and subsequent release of the sorbed ions to a stripping phase through electrochemical control of the specific binding processes. A remarkable degree of selectivity is shown for carboxylates (–COO–), sulfonates (–SO3−), and phosphonates (–PO3−2) over inorganic anions such as PF6− and ClO4− (separation factor >140 in aqueous and >3000 in organic systems), and between carboxylates with various substituents, based on differences in electronic structure and density of the adsorbates, beyond size, and charge. Our organometallic redox electrodes are a promising platform for targeting aqueous and organic systems requiring high separation factors and fast throughput, such as in the recovery of value-added products from organic synthesis and isolation of dilute yet highly toxic organic contaminants. The combination of spectroscopic experiments and quantum chemistry sheds light on a selective binding mechanism based on redox-enhanced hydrogen bonding between the cyclopentadienyl ligand and the carboxylate functional group, with broader implications for molecular design, supramolecular recognition, and metallocene catalysis.