Developing an approach for first-principles catalyst design: application to carbon-capture catalysis
Abstract
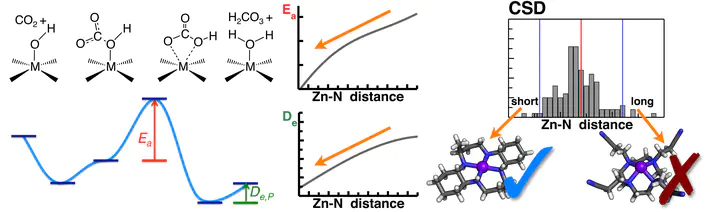
An approach to catalyst design is presented in which local potential energy surface models are first built to elucidate design principles and then used to identify larger scaffold motifs that match the target geometries. Carbon sequestration via hydration is used as the model reaction, and three- and four-coordinate sp2 or sp3 nitrogen-ligand motifs are considered for ZnII metals. The comparison of binding, activation and product release energies over a large range of interaction distances and angles suggests that four-coordinate short ZnII—Nsp3 bond distances favor a rapid turnover for CO2 hydration. This design strategy is then confirmed by computationally characterizing the reactivity of a known mimic over a range of metal–nitrogen bond lengths. A search of existing catalysts in a chemical database reveals structures that match the target geometry from model calculations, and subsequent calculations have identified these structures as potentially effective for CO2 hydration and sequestration.