Abstract
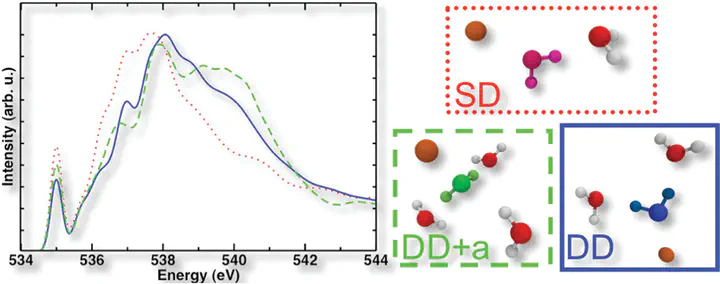
Both first-principles molecular dynamics and theoretical X-ray absorption spectroscopy have been used to investigate the aqueous solvation of cations in 0.5 M MgCl2, CaCl2, and NaCl solutions. We focus here on the species-specific effects that Mg2+, Ca2+, and Na+ have on the X-ray absorption spectrum of the respective solutions. For the divalent cations, we find that the hydrogen-bonding characteristics of the more rigid magnesium first-shell water molecules differ from those in the more flexible solvation shell surrounding calcium. In particular, the first solvation shell water molecules of calcium are able to form acceptor hydrogen bonds, and this results in an enhancement of a post-edge peak near 540 eV. The absence of acceptor hydrogen bonds for magnesium first shell water molecules provides an explanation for the experimental and theoretical observation of a lack of enhancement at the post-main-edge peak. For the sodium monovalent cation we find that the broad tilt angle distribution results in a broadening of postedge features, despite populations in donor-and-acceptor configurations consistent with calcium. We also present the reaveraged spectra of the MgCl2, CaCl2, and NaCl solutions and show that trends apparent with increasing concentration (0.5, 2.0, 4.0 M) are consistent with experiment. Finally, we examine more closely both the effect that cation coordination number has on the hydrogen-bonding network and the relative perturbation strength of the cations on lone pair oxygen orbitals.