Abstract
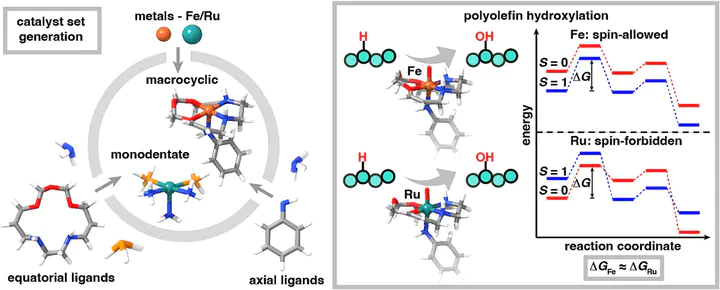
We performed a large-scale density functional theory comparison of polyolefin C–H hydroxylation trends across over 200 Fe and Ru catalysts that are identical except for their metal centers for the radical-rebound conversion of propane to propanol. We observed a strong spin-state dependence: higher-spin states had more favorable metal-oxo formation and isopropanol release in Ru catalysts, while hydrogen atom transfer (HAT) was more favorable in Fe catalysts. While the widely studied metal-oxo formation vs. HAT linear free-energy relationship held for Ru, it was more easily disrupted for Fe. Ru catalysts have a spin-forbidden C–H hydroxylation pathway, while Fe catalysts favor a spin-allowed, intermediate-spin pathway. Calculation of reaction coordinates on representative catalysts corroborated these spin–reactivity trends and showed comparable energetic spans for Fe and Ru analogues, as well as strong Brønsted–Evans–Polanyi relationships for both the metal-oxo formation and HAT steps, motivating expanded study of Fe catalysts.