Substrate Placement Influences Reactivity in Non-heme Fe(II) Halogenases and Hydroxylases
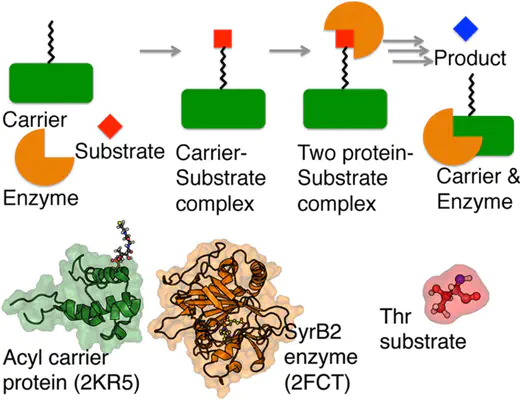
Abstract
We employ error-corrected density functional theory methods to map out the dependence of reactivity on substrate position for SyrB2, a member of a family of non-heme iron halogenases and hydroxylases that are only reactive toward amino acid substrates delivered via prosthetic phosphopantetheine arms. For the initial hydrogen abstraction step, the inherent flexibility of the phosphopantetheine molecule weakens the position dependence for both the native substrate (threonine for SyrB2) and alternative substrates. Over a 5 Å window of substrate positions, the tethered hydrogen abstraction step proceeds with nearly identical activation energies and donor-acceptor distances in the transition state. The propensity of a particular substrate toward halogenation or hydroxylation is found to depend strongly on the substrate placement following hydrogen abstraction, with deeper substrate delivery into the active (for native substrates) site favoring halogenation and shallower substrate delivery favoring hydroxylation.