Abstract
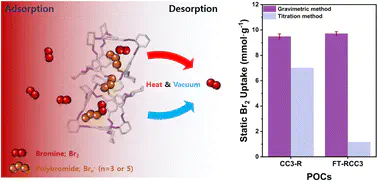
Highly volatile and toxic bromine (Br2) molecules can be utilized safely in various chemical processes when coupled with efficient separation systems. Herein, we present two different N-containing porous organic cages (POCs), covalent cage 3-R (CC3-R) and formaldehyde tied-reduced covalent cage 3 (FT-RCC3), for vapor Br2 capture under ambient conditions. They show outstanding sorption capacities (11.02 mmol g-1 and 11.64 mmol g-1, respectively) compared with previously reported adsorbents. Reversibility of the Br2 sorption process has been elucidated experimentally and computationally by identifying bromine species adsorbed at POCs and calculating their binding energies. The strong charge-transfer interactions between adsorbed Br2 and abundant N atomic sites of the host cages led to the dominant formation of polybromide species (Br3- and Br5-). Further host–guest interaction between POCs and polybromides determined the reversibility of the Br2 sorption process— showing partially reversible (>70% recovery) behavior for CC3-R and irreversible (<10% recovery) behavior for FT-RCC3, both of which were affected by the chemical and structural nature of different POCs. DFT calculations further indicate that the formation of carbocationic species (Br3- and Br5-) and HBr is energetically favorable within the cage, which is in good agreement with the experimental results. This work demonstrates that strong host–guest interactions are essential for highly efficient Br2 capture and storage performance.