Abstract
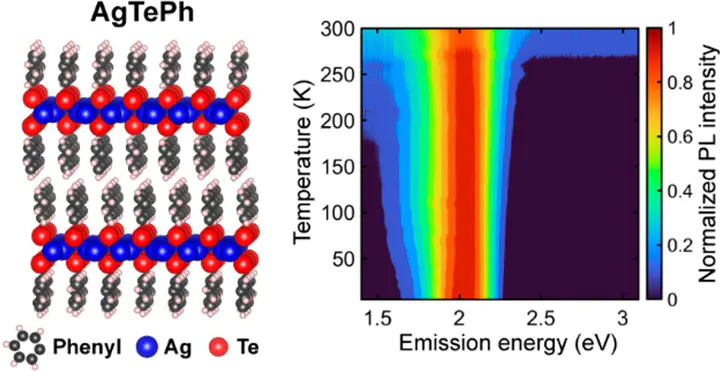
Silver phenylselenolate (AgSePh, also known as “mithrene”) and silver phenyltellurolate (AgTePh, also known as “tethrene”) are two-dimensional (2D) van der Waals semiconductors belonging to an emerging class of hybrid organic–inorganic materials called metal–organic chalcogenolates. Despite having the same crystal structure, AgSePh and AgTePh exhibit a strikingly different excitonic behavior. Whereas AgSePh exhibits narrow, fast luminescence with a minimal Stokes shift, AgTePh exhibits comparatively slow luminescence that is significantly broadened and red-shifted from its absorption minimum. Using time-resolved and temperature-dependent absorption and emission microspectroscopy, combined with subgap photoexcitation studies, we show that exciton dynamics in AgTePh films are dominated by an intrinsic self-trapping behavior, whereas dynamics in AgSePh films are dominated by the interaction of band-edge excitons with a finite number of extrinsic defect/trap states. Density functional theory calculations reveal that AgSePh has simple parabolic band edges with a direct gap at Γ, whereas AgTePh has a saddle point at Γ with a horizontal splitting along the Γ-N1 direction. The correlation between the unique band structure of AgTePh and exciton self-trapping behavior is unclear, prompting further exploration of excitonic phenomena in this emerging class of hybrid 2D semiconductors.