Visible-Light-Mediated Macrocyclization for the Formation of Azetine-Based Dimers
Abstract
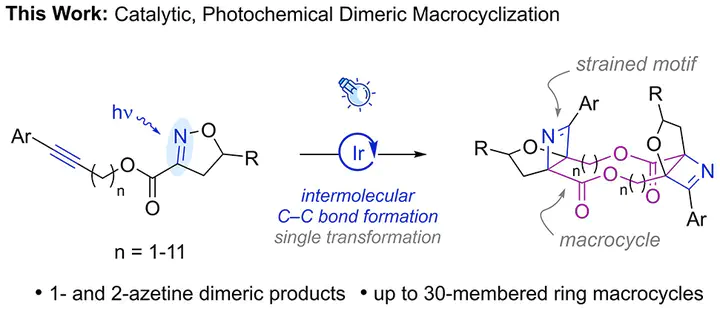
Macrocyclic dimeric lactones have pharmacological activities that make them attractive synthetic targets, but typical synthetic strategies employ an iterative approach to construct the macrocycle. Herein, we report a visible-light-mediated approach that enables facile access to 1- and 2-azetine-based dimeric lactones of up to 30-membered ring macrocycles. These products form via four consecutive triplet energy transfers for 1-azetine dimeric products and two consecutive triplet energy transfers for 2-azetine dimeric products. Computational investigations provide insights into the mechanism of this reaction, consistent with an unexpected initial intermolecular [2 + 2]-cycloaddition being preferred under nonstandard Curtin–Hammett conditions over the corresponding intramolecular reaction, which ultimately enables an efficient reaction pathway for macrocyclic dimerization.