Why Nonheme Iron Halogenases Do Not Fluorinate C–H Bonds: A Computational Investigation
Abstract
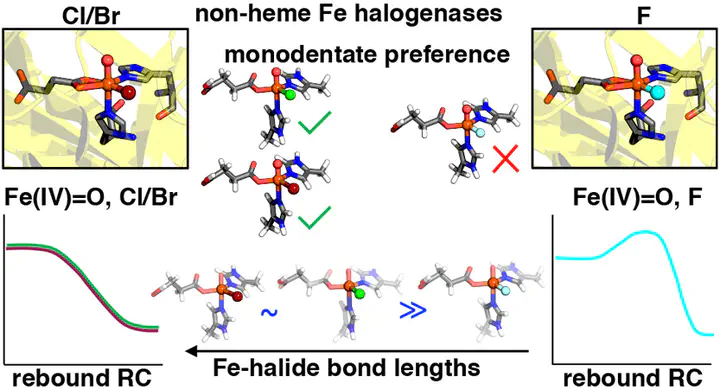
Selective halogenation is necessary for a range of fine chemical applications, including the development of therapeutic drugs. While synthetic processes to achieve C–H halogenation require harsh conditions, enzymes such as nonheme iron halogenases carry out some types of C–H halogenation, i.e., chlorination or bromination, with ease, while others, i.e., fluorination, have never been observed in natural or engineered nonheme iron enzymes. Using density functional theory and correlated wave function theory, we investigate the differences in structural and energetic preferences of the smaller fluoride and the larger chloride or bromide intermediates throughout the catalytic cycle. Although we find that the energetics of rate-limiting hydrogen atom transfer are not strongly impacted by fluoride substitution, the higher barriers observed during the radical rebound reaction for fluoride relative to chloride and bromide contribute to the difficulty of C–H fluorination. We also investigate the possibility of isomerization playing a role in differences in reaction selectivity, and our calculations reveal crucial differences in terms of isomer energetics of the key ferryl intermediate between fluoride and chloride/bromide intermediates. While formation of monodentate isomers believed to be involved in selective catalysis is shown for chloride and bromide intermediates, we find that formation of the fluoride monodentate intermediate is not possible in our calculations, which lack additional stabilizing interactions with the greater protein environment. Furthermore, the shorter Fe–F bonds are found to increase isomerization reaction barriers, suggesting that incorporation of residues that form a halogen bond with F and elongate Fe–F bonds could make selective C–H fluorination possible in nonheme iron halogenases. Our work highlights the differences between the fluoride and chloride/bromide intermediates and suggests potential steps toward engineering nonheme iron halogenases to enable selective C–H fluorination.