Polymer Networks with Cubic, Mixed Pd(II) and Pt(II) M₆L₁₂ Metal–Organic Cage Junctions: Synthesis and Stress Relaxation Behavior
Abstract
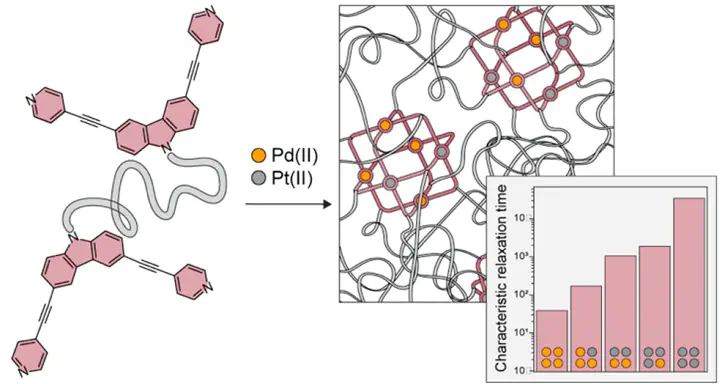
Metal–organic cages/polyhedra (MOCs) are versatile building blocks for advanced polymer networks with properties that synergistically blend those of traditional polymers and crystalline frameworks. Nevertheless, constructing polyMOCs from very stable Pt(II)-based MOCs or mixtures of metal ions such as Pd(II) and Pt(II) has not, to our knowledge, been demonstrated, nor has exploration of how the dynamics of metal–ligand exchange at the MOC level may impact bulk polyMOC energy dissipation. Here, we introduce a new class of polymer metal–organic cage (polyMOC) gels featuring polyethylene glycol (PEG) strands of varied length cross-linked through bis-pyridyl-carbazole-based M₆L₁₂ cubes, where M is Pd(II), Pt(II), or mixtures thereof. We show that, while polyMOCs with varied Pd(II) content have similar network structures, their average stress–relaxation rates are tunable over 3 orders of magnitude due to differences in Pd(II)- and Pt(II)-ligand exchange rates at the M6L12 junction level. Moreover, mixed-metal polyMOCs display relaxation times indicative of intrajunction cooperative interactions, which stands in contrast to previous materials based on point metal junctions. Altogether, this work (1) introduces a novel MOC architecture for polyMOC design, (2) shows that polyMOCs can be prepared from mixtures of Pd(II)/Pt(II), and (3) demonstrates that polyMOCs display unique relaxation behavior due to their multivalent junctions, offering a strategy for controlling polyMOC properties independently of their polymer components.